|
|
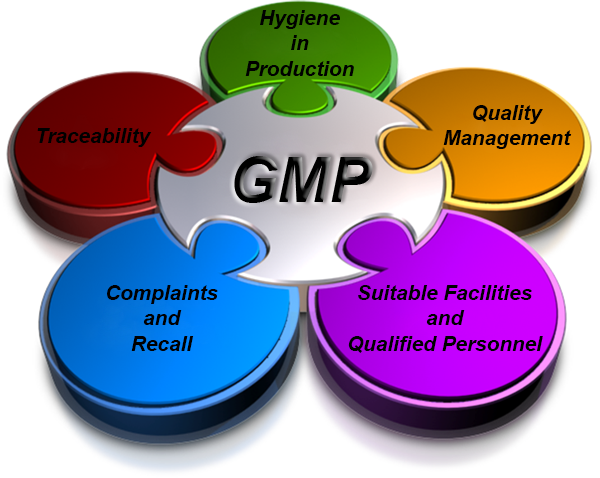
GMP
certificate
A good manufacturing practice (GMP) is a production and testing practice that helps to ensure a quality product. Many countries have legislated that pharmaceutical and medical device companies must follow GMP procedures, and have created their own GMP guidelines that correspond with their legislation.
• Manufacturing processes are clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
• Manufacturing processes are controlled, and any changes to the process are evaluated. changes that have an impact on the quality of the soap are validated as necessary.
• Instructions and procedures are written in clear and unambiguous language. (Good Documentation Practices)
• Operators are trained to carry out and document procedures.
• Records are made, manually or by instruments, during manufacture that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the soap was as expected. Deviations are investigated and documented.
• Records of manufacture (including distribution) that enable the complete history of a batch to be traced are retained in a comprehensible and accessible form.
• Complaints about marketed soaps are examined, the causes of quality defects are investigated, and appropriate measures are taken with respect to the defective soap.
GMP guidelines are not prescriptive instructions on how to manufacture products. They are a series of general principles that must be observed during manufacturing. When a company is setting up its quality program and manufacturing process, there may be many ways it can fulfill GMP requirements. It is the company's responsibility to determine the most effective and efficient quality process.
|